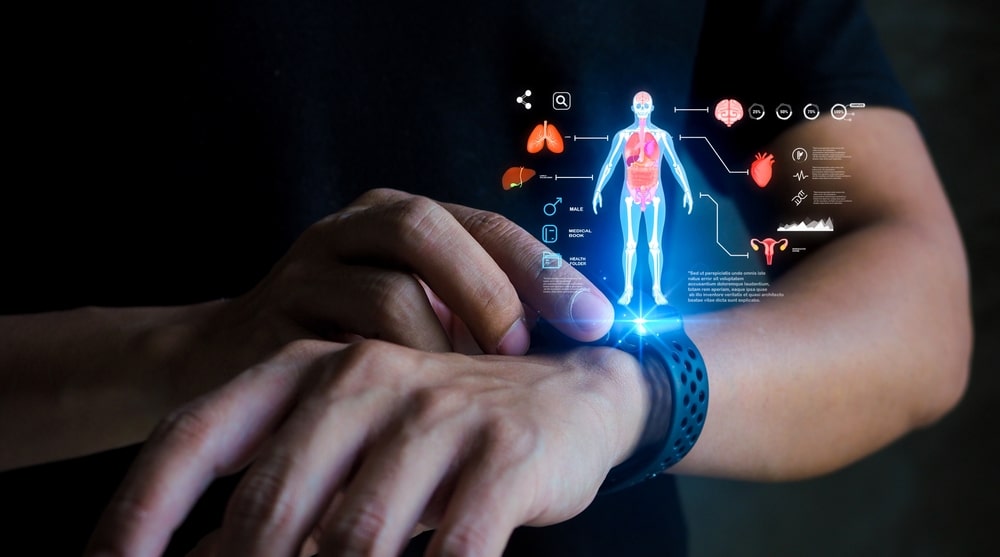
What’s New in Wearable Medical Devices?
In recent years, wearable medical devices have moved beyond simple fitness trackers to become sophisticated tools that provide real-time health data,


We are your expert strategic IVD and Device development partner streamlining the success of your envisioned product bringing your aspirations to life and promising a better health tomorrow.

Whether it’s an Over-the-Counter (OTC) or Point of Care (POC) in Vitro Diagnostic Device, we provide comprehensive solutions tailored to your product development and commercialization needs. Our expert team will guide you through the entire process, from initial concept to market launch, ensuring that each step is strategically planned and executed with precision.
Developing a medical device is a complex and multifaceted undertaking. We recognize the intricacies and challenges involved in navigating the regulatory landscape. Our expertise and experience allow us to guide you through every phase of the process, ensuring a smooth and efficient path to market.
Acenth is a full-service CRO providing Clinical Trial Management services for the Global IVD and Medical device Industry, while maintaining highest quality, ensuring strict adherence to your trial timeline and budget through collaborative approach.
From protocol design to project management, data analysis to Regulatory consulting and study submission to meeting with FDA, we provide solution from Inception to commercialization. We ensure your unique needs are met with our flexible approach and optimal support in predefined timelines.


Quality, commitment and trustworthiness are the core principles of Acenth.

In recent years, wearable medical devices have moved beyond simple fitness trackers to become sophisticated tools that provide real-time health data,

Artificial intelligence (AI) is transforming healthcare, and in vitro diagnostics (IVDs) are at the forefront of this revolution. At Acenth, Clinical

In today’s fast-evolving healthcare landscape, the integration of biostatistics into medical device and in vitro diagnostic (IVD) trials is indispensable. At Acenth, Clinical
Whether it’s an Over-the-Counter (OTC) or Point of Care (POC) in Vitro Diagnostic Device, we provide comprehensive solutions tailored to your product development and commercialization needs. Our expert team will guide you through the entire process, from initial concept to market launch, ensuring that each step is strategically planned and executed with precision.
With our extensive experience and in-depth knowledge of regulatory requirements, we can help you navigate the complex regulatory landscape with confidence. We understand that careful planning and the right support are critical to successfully bringing a product to market. That’s why we offer personalized assistance to help you identify and address potential challenges, streamline the approval process, and minimize time to market.
By partnering with us, you can be assured that your project will benefit from our commitment to quality, innovation, and efficiency. We are dedicated to helping you achieve your goals and accelerate your product’s entry into the market, ensuring that you can meet the demands of healthcare providers and patients alike.
Developing a medical device is a complex and multifaceted undertaking. We recognize the intricacies and challenges involved in navigating the regulatory landscape. Our expertise and experience allow us to guide you through every phase of the process, ensuring a smooth and efficient path to market.
We begin with strategic planning, working closely with you to outline a clear roadmap for development. This includes identifying key milestones, potential obstacles, and the most effective strategies to overcome them. Our goal is to provide a solid foundation that will support the entire development process.
Clinical trials are a critical component of medical device development. We assist in designing and implementing rigorous clinical studies that meet all regulatory standards. Our team ensures that your device is thoroughly tested and validated, providing the necessary data to support your regulatory submissions.
When it comes to regulatory submissions, we offer comprehensive support to ensure your device is classified and submitted correctly. Whether your device requires a Pre-Market Approval (PMA), a De Novo classification request, or a 510(k) notification, we have the expertise to guide you through the process. We help prepare all necessary documentation, respond to regulatory feedback, and address any issues that may arise during the review process.
By partnering with us, you benefit from our deep understanding of regulatory requirements and our commitment to helping you achieve a successful market entry. We are dedicated to supporting you at every step, from initial planning to final approval, ensuring that your medical device meets all necessary standards and reaches the market efficiently.