
Design Tips to Make Medical Devices Easier to Use
Medical device usability is increasingly critical for both patient safety and healthcare provider efficiency. At Acenth, we recognize the importance of designing devices that are


Medical device usability is increasingly critical for both patient safety and healthcare provider efficiency. At Acenth, we recognize the importance of designing devices that are
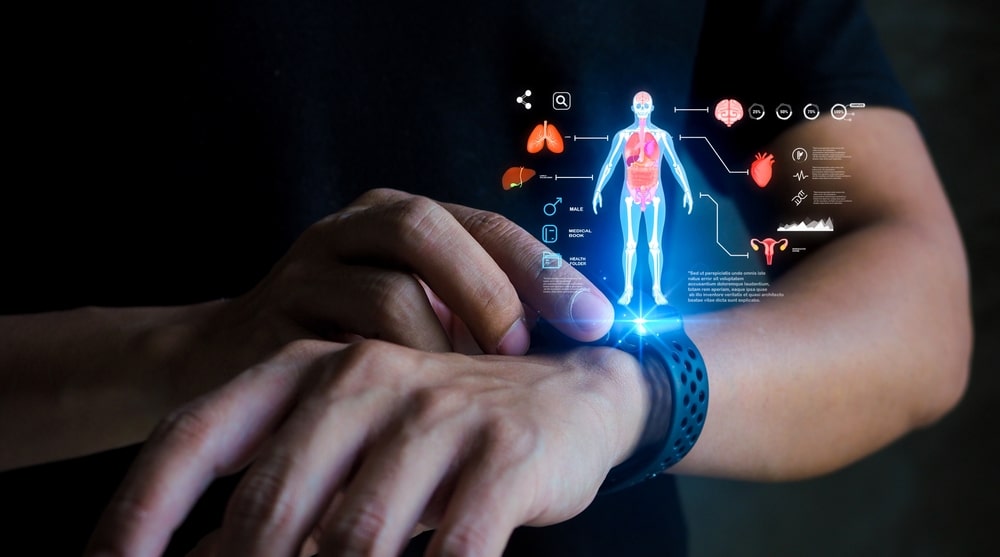
In recent years, wearable medical devices have moved beyond simple fitness trackers to become sophisticated tools that provide real-time health data, enabling physicians to make

Artificial intelligence (AI) is transforming healthcare, and in vitro diagnostics (IVDs) are at the forefront of this revolution. At Acenth, Clinical Research Organization (CRO), understanding

In today’s fast-evolving healthcare landscape, the integration of biostatistics into medical device and in vitro diagnostic (IVD) trials is indispensable. At Acenth, Clinical Research Organization (CRO), understanding

Clinical performance studies are essential for validating in vitro diagnostic (IVD) devices, ensuring that they provide accurate, reliable, and clinically meaningful results. For healthcare professionals

In vitro diagnostic (IVD) devices are crucial tools in modern healthcare, supporting diagnosis, treatment monitoring, and disease prevention. For healthcare professionals and researchers, understanding how

Medical device recalls are complex events that demand swift, organized, and ethical responses from healthcare professionals. For institutions and clinicians, ensuring patient safety while maintaining

Clinical trials are the cornerstone of medical innovation, and their integrity is essential to advancing safe and effective treatments. At Acenth, a trusted Clinical Research Organization

In the rapidly evolving world of healthcare, medical device labeling plays a crucial role in ensuring patient safety and regulatory compliance. At Acenth, working closely

In the evolving landscape of clinical research, decentralized clinical trials (DCTs) are gaining increasing attention, especially among healthcare professionals and doctors who play a crucial

Selecting a reliable Clinical Research Organization (CRO) is a pivotal step for healthcare professionals and doctors involved in in vitro diagnostic (IVD) or medical device

In today’s fast-evolving healthcare landscape, diagnostic tools are advancing to meet the increasing demands for speed, accessibility, and accuracy. Two such categories—Over-the-Counter (OTC) and Point-of-Care
The field of in vitro diagnostics (IVDs) is one of the fastest-evolving sectors in healthcare. With advances in genomics, digital diagnostics, and regulatory science, staying

In the fast-paced and highly regulated world of healthcare, clinicians and procurement specialists must ensure that the medical products they use meet the highest standards

In vitro diagnostics (IVDs) have long played a vital role in modern healthcare, offering the precision and speed needed to detect, monitor, and manage diseases.

Operating from the heart of the life sciences sector, Acenth collaborates with Clinical Research Organization (CRO) professionals to better understand the frameworks that support the

In vitro diagnostics (IVDs) are foundational to modern medicine. From genetic panels and infectious disease assays to cancer biomarkers and routine blood tests, IVDs inform

As medical technologies rapidly evolve, the clinical environment has become more reliant on advanced devices—from robotic surgical tools and implantable monitors to AI-driven diagnostics. For

The U.S. Food and Drug Administration (FDA) plays a foundational role in overseeing clinical research involving drugs, biologics, and medical devices. For healthcare professionals and

Medical product labeling serves as a critical conduit between manufacturers, regulators, and healthcare providers. For clinicians and researchers, the label is not just packaging—it’s a

Clinical trials are the backbone of medical innovation, leading to breakthrough treatments and therapies. However, designing and executing a successful trial presents numerous challenges, including

In vitro diagnostics (IVDs) have revolutionized clinical decision-making, allowing for early disease detection, targeted treatment planning, and efficient patient monitoring. However, choosing the right IVD

In vitro diagnostics (IVD) play a crucial role in modern healthcare by enabling early disease detection, monitoring treatment efficacy, and guiding clinical decision-making. From laboratory-based

Medical devices play a vital role in modern healthcare, ensuring that treatments, diagnostics, and patient care are effective and safe. However, obtaining certification and accreditation

In vitro diagnostic (IVD) devices have revolutionized healthcare by offering precise and timely diagnostic capabilities. However, as these devices become more connected through digital networks,

Acquiring and implementing medical devices is a significant investment for healthcare practices. From diagnostic tools to therapeutic technologies, these devices enhance patient care and streamline

In vitro diagnostic (IVD) devices play a vital role in modern healthcare by delivering accurate and timely diagnostic results. However, even the most advanced devices

The rapid advancements in medical device technology have revolutionized patient care, enabling precise diagnostics and efficient treatment plans. However, their adoption often hinges on a

In vitro diagnostics (IVDs) play a critical role in healthcare, enabling accurate disease diagnosis, monitoring, and patient care planning. With rapid advancements in IVD technology,

In vitro diagnostics (IVDs) play a vital role in modern healthcare, providing valuable insights into disease detection, patient monitoring, and treatment planning. However, the path

In the evolving landscape of healthcare, medical devices play a crucial role, providing advanced diagnostics, treatment options, and patient support. However, the economic factors surrounding

Medical devices are fundamental to modern healthcare, providing crucial support for diagnosis, treatment, and patient care. To maximize the effectiveness and safety of these tools,

Securing funding for clinical trials is critical to bringing new treatments and medical innovations to market. Understanding the various funding mechanisms and strategies available for

Conducting clinical trials for rare diseases presents a unique set of challenges that distinguish them from more common medical research. These challenges, however, are matched

In today’s complex healthcare landscape, effective risk management within regulatory services is more critical than ever. For healthcare professionals and organizations like Acenth, a Clinical

In the fast-paced world of healthcare, a medical product’s journey doesn’t end with its approval for market entry. Post-market surveillance (PMS) plays a pivotal role

In vitro diagnostics (IVD) are pivotal in modern healthcare, providing critical information for diagnosing and managing diseases. However, the pricing and reimbursement of IVDs significantly

In Vitro Diagnostic (IVD) equipment is critical in modern healthcare and clinical research, providing accurate diagnostic information that guides treatment decisions. For healthcare professionals and

Ethical standards in clinical research are the backbone of scientific integrity and patient safety. Understanding and adhering to these ethical principles is paramount for physicians
Placebos have long been a cornerstone of clinical trials, serving as essential tools in determining the efficacy of new treatments. Understanding the role and mechanisms

In the vast and intricate world of healthcare, clinical trials stand as pivotal elements in the regulatory decision-making process. As a Clinical Research Organization (CRO),

In the intricate world of medical product development, the journey from research and development (R&D) to market is fraught with challenges and regulatory hurdles. As

In the dynamic world of clinical research, the methods and protocols for data collection and analysis are foundational pillars. As a Clinical Research Organization (CRO),

In clinical research, understanding and anticipating patient experiences and challenges is not just a compassionate approach but a strategic imperative. As a Clinical Research Organization

In the intricate world of healthcare diagnostics, the quality and safety of In Vitro Diagnostic (IVD) devices are paramount. As a Clinical Research Organization (CRO),

In the ever-evolving landscape of healthcare, In Vitro Diagnostic (IVD) technologies stand at the forefront of innovation and precision. As a Clinical Research Organization (CRO),

The journey of medical device design and development is a fascinating and complex process, encompassing various stages from the initial concept to its final introduction

In the rapidly evolving healthcare landscape, medical devices have become indispensable tools, transforming patient care and clinical outcomes. Understanding the clinical significance of these devices

In the complex world of healthcare and clinical research, Clinical Research Organizations (CROs) face the perennial challenge of balancing safety and efficacy in regulatory decisions.

In the dynamic world of healthcare and clinical research, the role of regulatory bodies is pivotal. These entities, varying in structure and function across different